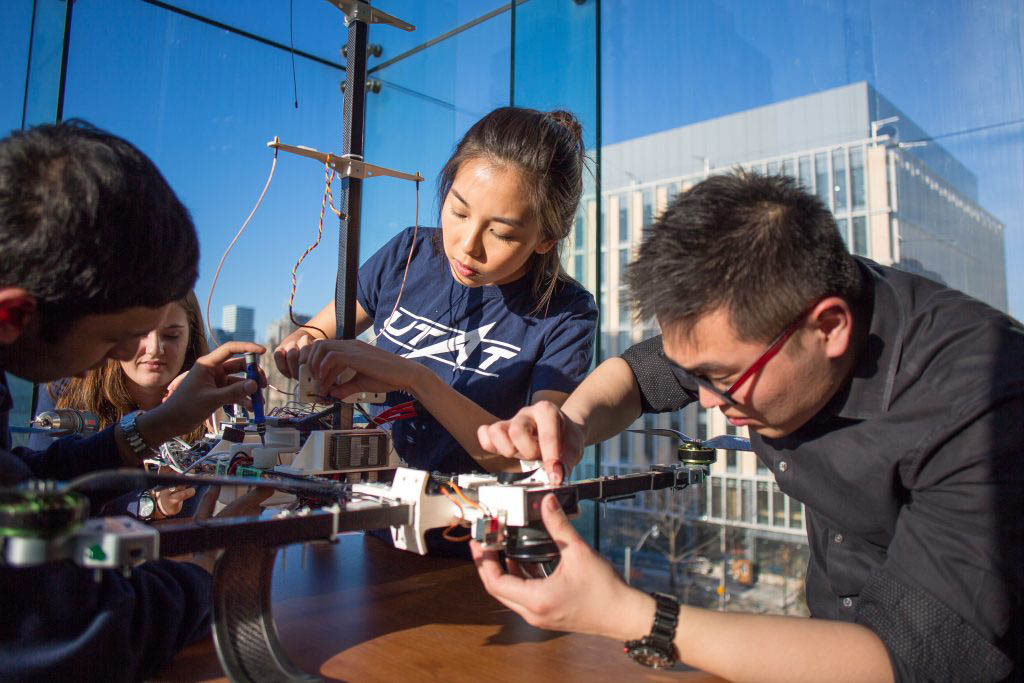
Advances in engineering and technology — from public sanitation to antibiotics — have enabled humans to live longer, healthier lives than ever before, yet many challenges remain.
Heart disease, neurodegenerative conditions and many types of cancer have few reliable treatments. The diseases that we can control still take far too many lives because of uneven distribution of technology around the world.
U of T Engineering researchers have a track record of success in working at the intersection of medicine, life sciences and engineering to prevent illness, treat disease and improve quality of life. We are creating tissue patches that can repair damaged hearts and other organs. We are optimizing surgical schedules and finding the best places to install artificial external defibrillators (AEDs). And we are developing low-cost, point-of-care diagnostics that enable developing countries to provide better care for everyone.
U of T has established strong partnerships with 10 world-class research and teaching hospitals in Toronto, including many within walking distance of our downtown campus. Together with doctors, medical researchers, policymakers and industry, we are helping people around the world live longer, healthier lives.
RESEARCH CENTRES & INSTITUTES
-
2018: Center for Advancing Neurological Innovation to Application (CRANIA™)
A $20-million partnership between the University Health Network and U of T, CRANIA aims to develop new neuromodulatory treatment strategies for neurological disorders like Alzheimer and Parkinson disease. It combines state-of-the-art facilities, training programs, and start-up development.
-
2015: Medicine by Design (MbD)
Created from a $114-million grant from the Canada First Research Excellence Fund, MbD is a collaboration between U of T Engineering, Arts & Science, Medicine and Pharmacy, as well as a number of affiliated hospitals. Its research takes a transdisciplinary approach to regenerative medicine and cell therapy to create therapies for diseases ranging from diabetes to cancer. Learn more about MbD.
-
2014: Translational Biology and Engineering Program (TBEP)
TBEP is U of T’s component of the Ted Rogers Centre for Heart Research (TRCHR), a world- class research centre established by the largest private health-care gift in Canadian history. Led by U of T Engineering professor Craig Simmons, TBEP leverages emerging technologies in tissue engineering, stem cell culture and cell signaling to develop therapies that can regenerate damaged hearts. Learn more about TBEP.
-
2008: Centre for Healthcare Engineering
The Centre focuses on leveraging systems engineering methods to optimize the delivery of health care, from surgical scheduling to medical technology, human factors research and health-care policy. Learn more about CHE.
-
2007: Southern Ontario Centre for Atmospheric Aersol Research (SOCAAR)
SOCAAR is focused on understanding the origins, characteristics, environmental impact, and human health consequences of atmospheric aerosols. Its principal investigators include researchers from U of T Engineering, Medicine, Arts & Science, the Dalla Lana School of Public Health and Environment Canada. Learn more about SOCAAR.
EXPERTISE
Selected areas of expertise in human health research at U of T Engineering
- Brain-Machine Interfacing
- Cell Manufacturing
- Disease Modeling & Therapeutics
- Health-care Engineering
- Heart Research
- Next-generation Medical Devices
- Public Health & Environment
- Pollution Exposure
- Regenerative Medicine
- Synthetic Biology
RESEARCH IMPACT
Mending broken hearts
After a heart attack (myocardial infarction), entire sections of heart tissue die. Currently, there is no way to replace this lost tissue, but that may be about to change.
Biomedical engineering professor MILICA RADISIC (left), Canada Research Chair in Functional Cardiovascular Tissue Engineering, and her team specialize in growing human tissues outside the body. They use a polymer that is both biocompatible and biodegradeable to create mesh-like scaffolds that can be seeded with human cells.
In a few days, the cells then begin to grow just as they would in a human: the team has produced pieces of heart tissue that beat with a regular rhythm and liver tissue that can metabolize certain biomolecules. Eventually, these tissue patches can be re-implanted
into the body to repair damage.
Even in their current state, these lab-grown constructs are tremendously useful for screening potential drug molecules and eliminating those that may have negative effects on heart or liver tissues. Radisic has created a startup, TARA Biosystems Inc., which has licensed the technology and is working with pharmaceutical manufacturers on this application.

Fighting eye infections with nanomedicine
Over 45 million people in the U.S. alone wear contact lenses to correct their vision. Contact lenses don’t fog up in the winter, won’t slip off your nose while running and are virtually invisible. But they are also an ideal surface for the proliferation of bacteria and other pathogens that can lead to serious eye infections.
The eye is also one of the most challenging organs for drug delivery. Separated from the rest of the body by multiple biological layers, the eye is protected from the external environment by tight cellular barriers and a tear defense system that dilutes any drugs which are applied.
Chemical engineering professor FRANK GU (centre), is designing particles at the nanoscale to detect bacterial pathogens and provide sustained drug delivery to the eye. As an NSERC Senior Industry Research Chair in Nanotechnology Engineering in partnership with one of the world’s largest pharmaceutical and healthcare companies, Gu is formulating gold nanoparticles of different sizes and geometries which can be incorporated into contact lens cases to detect and warn of pathogen contamination through a change in colour.
He is also designing drug-loaded nanoparticles that can stick to the mucous membranes at the surface of the eye and deliver their payload over a prolonged period of time, eliminating the need for repeated application and high doses.

Living heart valves from stem cells
Today, a baby born with a faulty heart valve can receive a synthetic replacement. But as the child grows, so does the size of the valve required. This means more surgical operations, increasing the risk of complication.
Professor CRAIG SIMMONS (middle), U of T Distinguished Professor of Mechanobiology and director of U of T’s Translational Biology and Engineering Program (TBEP), is working on a solution. Using stem cells from a child’s own umbilical cord, he and his team aim to grow replacement heart valves made of living tissue. These valves would grow as the patient grows, and the use of stem cells reduces the risk that the body will reject the implant. A similar approach could be taken with blood vessels and other tissues that need to grow over time.
Simmons’ work could also accelerate the arrival of personalized medicine by helping doctors choose which treatments will be most likely to benefit a particular patient. They are growing models of heart tissue and other organs from stem cells in the lab, which can be used to screen a library of drugs and select the most effective one for a given patient’s genetic makeup.

Point-of-care testing for remote communities
In many parts of the world, diagnosing, monitoring and screening for infectious diseases is a complicated task. Fully equipped medical laboratories are few and far between, and patients could take weeks to get a diagnosis, during which time their symptoms often worsen.
ChipCare is a company based on research from Professor STEWART AITCHISON and his graduate student JAMES DOU (above) in Electrical and Computer Engineering. The company has created the Polyvalent Analyzer (PAx) platform, which consists of a portable, easy-to-use analyzer and low-cost, disposable microfluidic cartridges. Based on a patented optical detection method, the system provides lab-quality results in as little as 15 minutes. The rugged design does not require refrigeration, enabling it to be deployed in remote locations without access to electricity.
While the initial design was created with HIV in mind, the platform can perform tests for a range of diseases. The company has raised more than $8 million in venture capital and is now scaling operations to bring its first-generation product to market.
“The discovery of pluripotent stem cells by UofT scientists James Till and Ernest McCulloch in the early 1960s opened up the possibility of using the body’s natural mechanisms to repair damage. Today, our tissue engineers are building on that legacy, developing new therapies for conditions from heart failure to neurodegenerative disease.”
PROFESSOR MICHAEL SEFTON
Michael E. Charles Chair in Chemical Engineering; Executive Director of Medicine by Design
THE FUTURE OF HUMAN HEALTH RESEARCH
Commercializing stem cell technology
In January 2016, Prime Minister Justin Trudeau visited U of T to announce the federal government’s $20-million grant to the Centre for Commercialization of Regenerative Medicine (CCRM) to establish and operate a new Centre for Advanced Therapeutic Cell Technologies. GE Healthcare also committed $20 million to the new centre.
CCRM is the commercialization partner of Medicine by Design. The new Centre will be the first such facility in the world to use a collaborative approach between research institutions and industry to solve cell therapy manufacturing challenges. By scaling up the manufacture of stem cells and derived products, the new partnerships will accelerate potential new therapies for a wide variety of conditions, including cancer, heart disease, and autoimmune diseases.
Building the bioproducts industry
In the future, biotechnology companies will produce designer cells, tissue and organs for a variety of purposes, from drug testing to treatment of degenerative disease.
How do you train highly qualified personnel to work in an industry that is still developing? One strategy is to teach them how to translate their own research into successful startups. That is the goal of the Training Program in Organ-on-a-chip Engineering and Entrepreneurship, headed by Professor MILICA RADISIC. Funded by an NSERC Collaborative Research and Training Experience (CREATE) grant, the program includes 11 primary investigators from U of T.
Another CREATE grant in the bioproducts space is Manufacturing, Materials and Mimetics (M3), headed by University Professor MOLLY SHOICHET, Canada Research Chair in Tissue Engineering. M3 focuses on cell manufacturing, biomaterials and tissue mimetics. In partnership with the CCRM, the program will produce graduates with the scientific and business acumen to advance this knowledge to industrial application.
SPECIALIZED EDUCATIONAL OFFERINGS IN HUMAN HEALTH
The Institute of Biomedical Engineering (BME) — a multidisciplinary research community of engineering, medicine and dentistry investigators — offers research-based graduate programs at both the Master’s and Doctoral levels, as well as a one-year Master of Engineering (MEng) that focuses on the design of biomedical devices.
At the undergraduate level, engineering students can minor in Biomedical Engineering, and Engineering Science students can major in Biomedical Systems.
LATEST HUMAN HEALTH STORIES
OUR INNOVATION CLUSTERS
U of T Engineering has the breadth and depth of research excellence as well as the capacity to effect global change across these key domains.
OUR EXPERTS
Find the U of T Engineering researchers with the expertise to solve your most complex challenges

LEADING INNOVATION STARTS HERE
DOWNLOAD PDF
Connect with us to discuss how a partnership with U of T Engineering can benefit your organization.